FDA-Approved
Insurance-Covered
Fast-Acting
How does Spravato Work?
Spravato is made from a derivative of ketamine called Esketamine (S-ketamine). This active ingredient sets off a chain of biological processes that boost the neurotransmitter Glutamate in regions of the brain associated with mood.1
Depression is associated with a decrease in Glutamate, which affects the brain’s overall functioning.
Studies have shown that activating Glutamate signaling can boost neuroplasticity to help the brain restore synaptic connections that may have been impacted by depression. The more plastic our brains are, the better we are able to respond to stress.
Depression is considered treatment-resistant once someone does not respond to at least two different antidepressants. 2

30% of people with depression do not respond to antidepressants.3
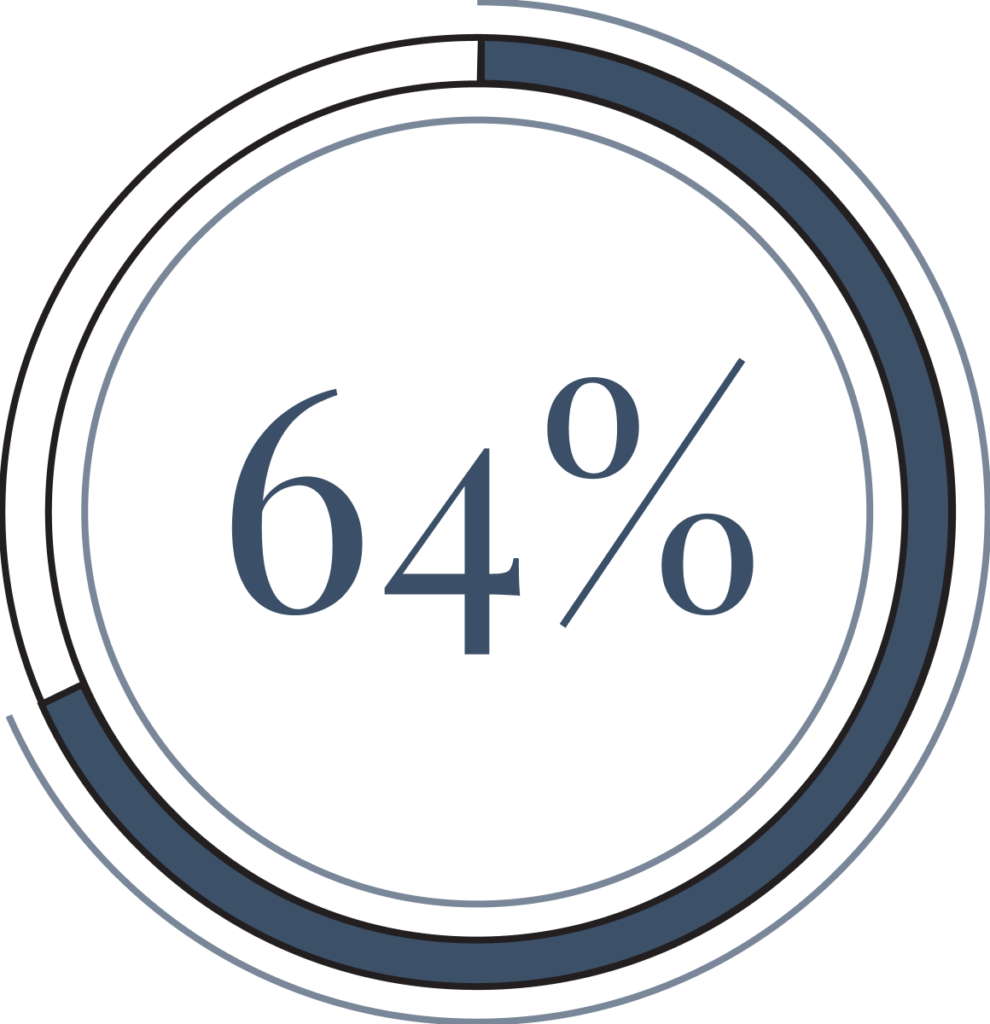
64% of patients with treatment-resistant depression reported a significant reduction in depressive symptoms after taking Spravato for 3 months.4
Spravato Treatment Timeline
Weeks 1-4
Weeks 5-8
Weeks 9+
What To Expect During Spravato Treatment

Step 1
Our team will show you how to self-administer the nasal spray.
Step 2
Relax in our office for up to two hours while we monitor for side effects.
Following your treatment you may experience feelings of dissociation, as though you are able to step outside of yourself and view your life from a new vantage point. Or you may experience visual or auditory hallucinations. These side effects typically wear off within two hours.
*Spravato is not a take-home medication. It must be taken in-office, under the supervision of Hudson Mind’s clinical team.
Common Questions
What are some common side effects?
- Dizziness
- Nausea
- Numbness
- Increased blood pressure
- Anxiety
- Lack of energy
- Spinning sensation
- Feeling sedated
What are the contraindications for Spravato?
- Aneurysmal vascular disease (including thoracic and abdominal aorta, intracranial, and peripheral arterial vessels) or arteriovenous malformation
- History of intracerebral hemorrhage
- Hypersensitivity to esketamine, ketamine, or any of the excipients
How do I pay for Spravato treatment?
- Insurance
Depending on your carrier and plan, your treatment may be covered by insurance. - Cash Pay
Cash pay is an option if your insurance does not cover your treatment. Please contact for pricing.
Is Spravato safe and/or well-tolerate?
Spravato is considered a safe and well-tolerated antidote to depression. However, before starting treatment it is important to give your healthcare provider a full list of current conditions and medications, as Spravato may interact with certain prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I prepare for treatment?
We recommend you avoid eating two hours before and drinking liquids 30 minutes before your treatment to minimize the possibility of nausea and vomiting.
Can I drive after my treatment?
Spravato may cause sleepiness and dizziness, so it is not safe to drive or operate machinery after your treatment. We advise waiting a full day (following a restful sleep) before driving.
- https://physicians.mountsinai.org/news/five-things-to-know-about-esketamine
- Gaynes BN, Lux L, Gartlehner G, Asher G, Forman-Hoffman V, Green J, Boland E, Weber RP, Randolph C, Bann C, Coker-Schwimmer E, Viswanathan M, Lohr KN. Defining treatment-resistant depression. Depress Anxiety. 2020 Feb;37(2):134-145. doi: 10.1002/da.22968. Epub 2019 Oct 22. PMID: 31638723.
- Zhdanava M, Pilon D, Ghelerter I, Chow W, Joshi K, Lefebvre P, Sheehan JJ. The Prevalence and National Burden of Treatment-Resistant Depression and Major Depressive Disorder in the United States. J Clin Psychiatry. 2021 Mar 16;82(2):20m13699. doi: 10.4088/JCP.20m13699. PMID: 33989464.
- https://www.google.com/url?q=https://www.janssenscience.com/products/spravato/medical-content/real-world-evidence-effectiveness-and-safety-of-spravato-therapy&sa=D&source=docs&ust=1695330142650904&usg=AOvVaw3Fj2C8YBCa2i0FOKVZGjXp
- https://www.spravatohcp.com/faq